The highly successful parasitic tapeworm known as Taenia solium has long been evidenced in human history, with ancient Greek and Chinese recordings. The Greeks correctly recognized the association of widely common intestinal tapeworms with the ingestion of cyst-laden pork. They referred to these “hailstones” in meat as cysticerci (which we still refer to them today), meaning “bladder-tails”.
Today, however, the parasite is closely associated with areas of poverty and poor sanitation practices in its only known definitive host, the human. Although the parasite is present worldwide, it is essentially eliminated in modernized civilizations due to advanced infrastructure and sanitary practices/strict food regulations/inspections (i.e. pigs no longer ingesting human feces containing eggs, and subsequently developing cysticerci which could then be ingested by under-cooked-pork-eating humans, thus perpetuating the parasite’s life cycle). However, T. solium infections are increasing in the United States, specifically due to more prevalent migratory practices of people indigenous to other countries where these infestations are prevalent. With this being said, pork should ALWAYS be thoroughly cooked to at least 160° F. I know (from one upsetting personal experience) there are some of you out there who get pleasure out of pinching off a taste of raw ground meat before cooking it and SHAME ON YOU. WHY? I would genuinely like to hear your perspective. Anyways, there’s my selfish PSA for the week.
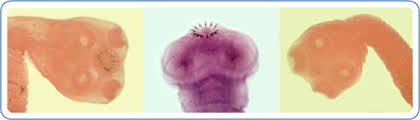
T. solium is known in humans as the “pork tapeworm”, as the organism, seen above, is transmitted by ingesting tapeworm larval cysts known as cysticerci in the metacestode stage present in under-cooked, infected pork. The tapeworm parasitizes the gut of the human definitive host causing eggs to be shed in the stool which can then infect themselves and others with another, more serious form of the disease in which the human is then also serving as the intermediate host. What a double whammy! Generally, tapeworm infections are mild and can go unnoticed for long periods, but if the eggs of the tapeworm are ingested by humans or pigs (pigs usually serve as the intermediate host), this can cause an infection known as cysticercosis in which the eggs develop into larvae and form cysts within various organs and tissues. When these invasive larvae enter the central nervous system they can cause dangerous neurological symptoms in humans (neurocysticercosis), including epileptic seizures, which can be fatal.

So how does a parasite sustain this level of success over thousands of years? Regarding parasitic tapeworms and most other successful parasites, in general, they are noted to typically never kill their host, as they only take just enough to allow them to thrive, grow, and have their host remain alive while shedding/spreading eggs in their stool (in this case). The complex coevolution of these two distantly related species (tapeworm and human) is astounding in terms of molecular adaptations, as even currently, different genotypes of T. solium differ regarding which part of the world it is from, and can be divided into two distinct genotypes – Asian/African and Latin American. Furthermore, molecular profiling of a worm from a traveling patient can allow for professionals to trace back to what region of the world the infection was initially acquired.
Individual patients can also exhibit different responses to genetically/geographically similar worms. This differing host response might be what is responsible for the variations noted in worms of different regions of the world. This would also explain different host responses to the same infestations.
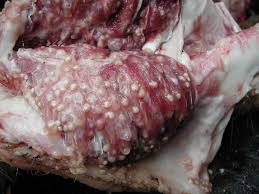
The cysticercus, seen above, must survive in the muscle for weeks to months, so it is not surprising that the cysts have developed elaborate mechanisms for evading the pig’s host response. By evolving to evade the host’s immune response in various ways that are just recently beginning to be better understood, cysticerci within swine muscle tissue have very little to no surrounding inflammation. Likewise, the worm within the gastrointestinal tract of a human host has also developed elaborate means of evading destruction by the body’s immune system. The parasite’s paramyocin binds to a complement component of the innate immune system, inhibiting activation. The parasite also secretes a serine protease inhibitor to accomplish the same inhibition.
It has been shown that humans exhibit a scarce or even absent immune response to the ingestion of T. solium eggs, and by the time the body has mounted a sufficient immune response, the the parasite has already begun to transform to the more resistant metacestode form (cysts within tissue). The parasite actively prevents this inflammatory response, which prolongs its survival in the host and continues this suppression until the cysts begin to degenerate within the body, which is what causes the severe onset of inflammation and symptoms. However, cysticerci do have the capability of gently stimulating the body’s immune system in order to use cells as a protein source, while forming taeniaestatin, among other molecules, that interfere with the cells of the innate immune system, essentially paralyzing our natural immune response.

Perhaps the most fascinating article associated with this topic comes from Aguilar-Díaz et al. in which the team explores how host-parasite coevolution (as well as gender-associated selection to this infection) has occurred over time. Moreover, their findings provided evidence on the crosstalk between the host and parasite at both molecular and evolutionary levels. Specifically, the host’s progesterone has a direct effect on the growth of T. solium cysticerci, among other parasitic factors, as well as progesterone mediation by a steroid binding parasite protein. The parasite was shown to exhibit a progesterone binding protein, initially acquiring this adaptation by either horizontal gene transfer, evolution by mimicry, or, perhaps, from common ancestral genes. Much remains to be understood about this fascinating host/parasite relationship. Studies such as these are vital in understanding the complex interactions so that we may, ultimately, provide better anti-helminth drugs to avoid these potentially dangerous infections.

Sources and Supplemental Information
Bobes, Raul & Fragoso, Gladis & Fleury, Agnes & García-Varela, Martín & Sciutto, Edda & Larralde, Carlos & Laclette, Juan. (2014). Evolution, molecular epidemiology and perspectives on the research of taeniid parasites with special emphasis on Taenia solium. Infection, Genetics and Evolution. 23
Muthukumar N. Commentary: Neurocysticercosis: Evolution of our understanding. Neurol India 2017;65:885-7
NAKAO, M., OKAMOTO, M., SAKO, Y., YAMASAKI, H., NAKAYA, K., & ITO, A. (2002). A phylogenetic hypothesis for the distribution of two genotypes of the pig tapeworm Taenia solium worldwide. Parasitology, 124(6), 657-662
Pajuelo MJ, Eguiluz M, Roncal E, Quiñones-García S, Clipman SJ, Calcina J, et al. (2017) Genetic variability of Taenia solium cysticerci recovered from experimentally infected pigs and from naturally infected pigs using microsatellite markers. PLoS Negl Trop Dis 11(12): e0006087
White AC Jr, Tato P, Molinari JL. Host-parasite interactions in Taenia solium cysticercosis. Infectious Agents and Disease 1992;1:185-93
White AC Jr, Robinson P, Kuhn R. Taenia solium cysticercosis: hostparasite interactions and the immune response. Chemical Immunology 1996; 7:66.
https://www.cdc.gov/parasites/cysticercosis/epi.html
https://www.who.int/news-room/fact-sheets/detail/taeniasis-cysticercosis
White AC Jr, Tato P, Molinari JL. Host-parasite interactions in Taenia solium cysticercosis. Infectious Agents and Disease. 1992 Aug;1(4):185-193.
Gutierrez-Loli, Renzo & Orrego, Miguel & Sevillano Quispe, Oscar & Herrera-Arrasco, Luis & Guerra-Giraldez, Cristina. (2017). MicroRNAs in Taenia solium Neurocysticercosis: Insights as Promising Agents in Host-Parasite Interaction and Their Potential as Biomarkers. Frontiers in Microbiology. 8. 1905. 10.3389/fmicb.2017.01905.
Aguilar-Díaz H, Nava-Castro KE, Escobedo G, et al. A novel progesterone receptor membrane component (PGRMC) in the human and swine parasite Taenia solium: implications to the host-parasite relationship. Parasit Vectors. 2018;11(1):161. Published 2018 Mar 9. doi:10.1186/s13071-018-2703-1
Hélène Carabin, Linda Cowan,Theodore Nash, A. Lee Willingham. Estimating the Global Burden of Taenia solium Cysticercosis/Taeniosis. WHO/FAO Collaborating Center for Parasitic Zoonoses The Royal Veterinary and Agricultural University Frederiksberg, Denmark